What's Happening Archive
The PMEL Ocean Molecular Ecology group collaborated with the PMEL Earth-Ocean Interactions Program (EOI) in March-April 2023 in an ocean exploration effort to identify and describe new hydrothermal sites along the Mid-Atlantic Ridge. The expedition took place on the Schmidt Ocean Institute's new vessel the RV Falkor (too).
The group found a suite of never before documented hydrothermal vent fields. Sean McAllister, a member of Ocean Molecular Ecology, participated through the collection of environmental (e)DNA samples in transects over the sites of hydrothermal venting, ultimately providing a picture of biodiversity of microbial to macrofauna communities. Individual tissue samples were also taken to enhance reference barcode databases and improve eDNA taxonomic assignment accuracy. In addition, while underway, Sean was able to extract and sequence the eDNA samples collected using a Nanopore long-read DNA sequencer to generate near real-time characterizations of the microbial and invertebrate communities at the hydrothermal vent sites. Visit the expedition website, view the cruise log which includes science blog posts and a highlights, and check out the dive stream for all ROV dives to learn more about these exploratory efforts.

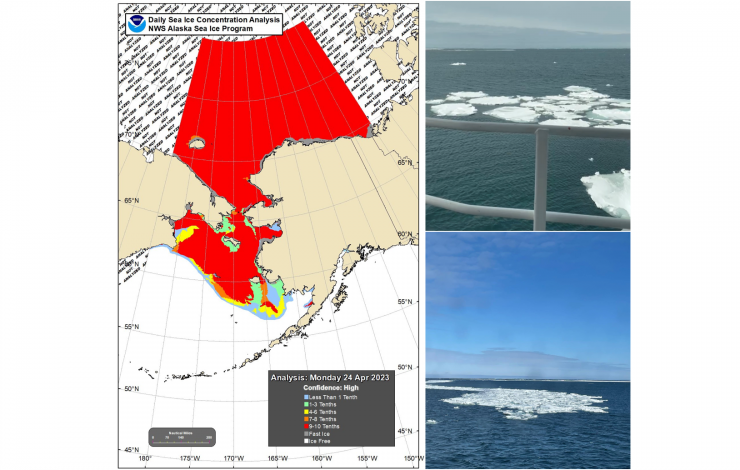
April 21 - May 8 - NOAA PMEL oceanographers and colleagues continue leading an annual effort to collect key data in understanding the Bering Sea. This important research cruise provides key insights to monitor events such as sea-ice loss and the cold pool in the region and how these are impacting the Arctic ecosystem. This year’s cruise started out a bit different!
For the first time since 2012, the ice extent in the Bering Sea is impacting the survey and researchers had to alter their cruise plan as the ice is at and around several of the mooring and sampling sites. While not thick ice, lead scientist and NOAA oceanographer Phyllis Stabeno was surprised. “I did not expect to see ice this late on the shelf,” she said. In this region, ice arrives in the Bering Sea in the fall and typically melts and recedes in spring, - limiting when research vessels can be in the area.
2012 was a record breaking year. The melt season of 2012 started out at a sluggish pace. Around mid-April, sea ice extent was close to the 1979–2000 average for that time of year (the maximum ice extent typically occurring in March). However, soon after that the decline began to accelerate rapidly.
Stabeno took this as a unique and unexpected opportunity to safely sample around the ice edge. Most vessels are not made to break ice - but they can go near this melting ice region. As the ice continues to melt, the science team will resume their planned research.
This spring mooring cruise brings together scientists from NOAA’s PMEL and Alaska Fisheries Science Center, the University of Washington, US Fish and Wildlife, and the University of Alaska. While aboard the NOAA Ship Oscar Dyson, the scientists will service a biophysical mooring array. They will also collect water samples of conductivity (salinity), temperature, depth (CTD) profiles, zooplankton, ichthyoplankton, nutrients and chlorophyll samples. As well as conduct collaborative research including on harmful algal blooms, omics, and zooplankton machine learning. Results from these observations and experiments will help describe important ecosystem linkages among climate, plankton, fishes, birds and mammals.
EcoFOCI will be field testing and using several innovations this spring. Innovations and technologies such as these aim to enhance shipboard and mooring research with advanced and increased data collection. These include the deployment of a modified ‘high-latitude’, more robust surface mooring at M2 and a shallow-water glider. This is the 29th consecutive year the M2 mooring will be deployed. In 2022 a combination of the pandemic, sea ice, and a storm provided researchers a new perspective from NOAA’s longest operating biophysical mooring site in the US Arctic. Learn more about that in a NOAA Story Map (https://storymaps.arcgis.com/stories/1b413464b13c4aa381b48ecd5c89ed50).
This is the first of five NOAA EcoFOCI program research cruises planned between April and October in the Alaska region.
Learn more about mooring arrays and the EcoFOCI spring cruise on NOAA Fisheries 2022 blog.
Hello! My name is Chloe Rabinowitz, and I am an undergraduate research intern through a joint collaboration between the University of Washington (UW) EarthLab and NOAA PMEL Ocean Molecular Ecology group. This summer, I sampled zooplankton from the Salish Sea and then proceeded to extract DNA and sequence species of interest. The purpose of my work has been to publish whole mitochondrial genomes to the national nucleotide database (NCBI) to enhance our understanding of zooplankton biodiversity in the region. Sequencing the full mitogenome enriches the coverage of zooplankton species by ensuring there is a reference sequence regardless of the targeted DNA amplicon. Additionally, information gained by assembling the mitochondrial genome can provide significant insight in understanding the evolutionary history of a species.
At the beginning of my internship, I worked with researchers on a Washington Ocean Acidification Center (WOAC) cruise. As we visited long-term monitored sites around the Salish Sea, I conducted vertical and oblique net tows into the ocean to a predetermined depth and pulled them up through the water to collect zooplankton samples. When I returned to land, I analyzed the zooplankton samples from previous WOAC cruises. Participating in a sampling effort allowed me to see the entire step-by-step process of my research. For the next few weeks of my internship, I worked with mentors in the Keister Lab at UW to fine-tune my zooplankton taxonomy skills. Using a microscope and my trusty forceps, I pulled out eight species of interest from the samples, seven copepods and one amphipod. Zooplankton, such as copepods and amphipods are important to the marine food web as they are the foundational food/energy source for marine invertebrates, fish, mammals, and most things in between. The health and functionality of any marine ecosystem is directly impacted by the health, diversity, and abundance of zooplankton.

Once I identified and separated out species (even up to 2000 individuals for one species), I brought my samples to the NOAA Western Regional Office to begin my work with the PMEL ‘Omics Group. In the ‘Omics lab, I photographed each species of interest, so we had physical documentation of the specimens extracted. Then, due to their small size, we used two different extraction methodologies to determine which would result in the highest DNA yield. Next, we checked the quality and quantified how much DNA was extracted from each organism with PCR gel electrophoresis and a Qubit fluorometer. We utilized a Nanopore long-read DNA sequencer to sequence the mitochondrial genome of the amphipod species, Cyphocaris challengeri. In addition, C. challengeri and four copepod species with the highest DNA yields post-extraction, were sent for Illumina NGS sequencing. With the results of the full mitochondrial genome sequences, we will gain a better understanding of the ecology and regional impacts of this species. The information we obtain will be published to enhance scientific knowledge of their ecology and evolution, improve public awareness, and allow us to detect and monitor their presence in Puget Sound.
While zooplankton might seem like small organisms, their impact is vast. Through my work during this internship and the passion/dedication I’ve seen from my mentors, it is clear that genomics is a critical tool that allows us to understand the deep complexity of important creatures in marine ecosystems that would otherwise potentially go unnoticed.
Hello, my name is Owen Yazzie, and I am a Cooperative Institute for Climate, Ocean, and Ecosystem Studies (CICOES) undergraduate research intern working at the University of Washington. This summer, I assisted the ‘Omics Group with an ongoing project in the Olympic Coast National Marine Sanctuary (OCNMS), off the outer coast of Washington state. The Ocean Molecular Ecology group is working with OCNMS to deploy autonomous environmental (e)DNA samplers in the sanctuary to study how biodiversity changes during and surrounding hypoxic events. During the 2022 field season, the group has two planned deployments scheduled, with automated samplers out for a month-long window at two sites (Teahwhit Head and Cape Elizabeth). These automated samplers collect a total of 24 samples spaced every 36 hours, eliminating the need for costly in-person sampling. I participated in the summer recovery cruise at OCNMS, and that opportunity allowed me to gain hands-on experience in the lab and in the field. Aside from the lab/research experience, there were other first time experiences for myself, such as seeing marine life outside of an enclosure.

In preparation for fieldwork, I assisted with packing gear and sterilizing equipment required for eDNA sampling. Environmental DNA samples are prone to contamination, so it was important we carefully sterilize the bottles and tubing used in the field. On the two-day recovery cruise, aboard the OCNMS vessel, the R/V Storm Petrel, we recovered both automated samplers and collected water samples using a CTD niskin array at Teahwhit Head and Cape Elizabeth. To generate occupancy models, which account for imperfect detection of organisms and to determine the probability of their true presence or absence, we collected 10 water samples near the automated sampler just prior to recovery to supplement a sliding window analysis. To do this, we started by lowering a CTD niskin array to the depth of the sampler, niskin bottles were then closed to capture water at that depth, and once returned to the ship deck, we collected the water samples in our sterilized bottles. Later we filtered the eDNA from those samples, while maintaining sterile technique to prevent contamination. I assisted with multiple steps of the filtration process, including the preservation of the eDNA samples in ethanol. I also worked alongside the OCNMS crew to recover the automated samplers. These moorings included an acoustic release mechanism, which allowed for the recovery of the entire mooring and ensured we did not leave anything behind. On both days, I helped collect the 24 samples (i.e., filters) from the automated samplers, while maintaining sterility during this process. Assisting with this project was a very rewarding experience that I am grateful to have taken part in.
The Ocean Molecular Ecology (OME) group received a major upgrade to their lab infrastructure, a portable lab in a shipping container, commonly referred to as a lab van. This past week, the lab van was installed at NOAA's Western Regional Office and will serve as a clean lab for the group to process environmental (e)DNA samples. The lab van significantly increases the in-house sample processing capabilities for the group and decreases the cost of outsourcing several steps of the eDNA library prep process. The 'Omics Group was previously limited in their ability to process eDNA samples due to a lack of appropriate workspace, so the scientists are eager to begin using this dedicated space.
Environmental e(DNA) samples are highly prone to contamination risks; therefore, samples must be processed in designated clean areas kept separate from other types of molecular work and free from potential airborne contaminants. One of the main sterilization features of the Omics lab van is the UV lighting system that can irradiate the entire working space after each use. In addition, the built-in HEPA filters work to eliminate the risk of airborne contaminants. The workspace includes two Class II Biosafety cabinets, one for DNA extraction and one for PCR amplifications and sequencing library prep. The biosafety cabinets are enclosed, ventilated laboratory workspaces used for processing low biomass molecular samples. Other key features of the Omics lab van include a reverse osmosis (RO) system, ample secure storage, and a double entryway to maintain a sterile/positive pressure environment. The 'Omics Group employs eDNA to monitor biodiversity shifts in the Northeast Pacific and Alaska waters. Their biological data is then comparatively examined with physical and chemical oceanographic parameters to study ecosystem biodiversity and ocean health. Once their lab van is officially commissioned, the 'Omics Group will immediately take advantage of this new resource and eDNA samples for sequencing from the 2021 and current 2022 field seasons.

My name is David Garcia-Prieto, and I’m interning in PMEL Ocean Molecular Ecology group for the summer. My current funding source, NOAA-LMRCSC, is part of NOAA’s Educational Partnership Programs & Minority Serving Institutions. This program supports doctoral students through graduate school and provides students with professional experience. As part of the program, NOAA’s Experiential Research and Training Opportunities (NERTO) gives students the opportunity to work alongside NOAA scientists for a 12 week internship. The internship allows me to gain experience in a government facility and to familiarize myself with NOAA culture, all while gaining more practical scientific experience. When searching for a home base for my NERTO internship, I found that ‘Omics Lab does genomic work complementary to my Ph.D. research. We are interested in using environmental DNA (eDNA) as a tool to study the microbial and zooplankton communities in seawater and their response to changing physical ocean conditions.
At the beginning of the I performed literature reviews, familiarized myself with the lab's protocols & SOPs, gained hands-on experience with PCRs, and worked in RStudio. I then had the privilege of participating in a 5-day research cruise around the Salish Sea. The cruise was one of the Washington Ocean Acidification Centers’ (WOAC) expeditions to help monitor the Puget Sound’s health by taking measurements like dissolved inorganic carbon (DIC) and oxygen content, things that can impact microbe and zooplankton populations. WOAC is a collaborative effort between U.W. scientists and interns to sample and analyze resulting data. Amongst other things, WOAC provides water quality monitoring of six shellfish hatcheries and rearing areas. The monitoring helps inform hatchery farmers when seawater requires treatment due to changing ocean chemistry.

We sailed aboard the R/V Rachel Carson where I collected 1-liter water samples from 11 sites at various depths (surface, middle and near bottom) of the water column to look at microbial and zooplankton community composition through eDNA. After collecting the samples, I filtered the water samples across a small filter in a sterile environment with a portable peristaltic pump. These filters catch most organic material, including microbes. Next, DNA was preserved with ethanol and stored in a freezer. Back in the lab, we extracted the DNA from these filters, PCR amplified target markers, and quantified the product. With a Nanopore-long read DNA sequencer, the DNA was sequenced, and the resulting data was run through an RStudio pipeline which helped visualize the recovered community composition.

Orcas feeding in the Drayton Passage, near Olympia, WA
This research is important as it allows us to understand the microbial and zooplankton composition of the water column as they are the base of the ecosystem. Microbes and other small, “simple” life forms are some of the first to acclimate to a changing environment, so we can use them as a biomarker to monitor a changing system. For my project, we are interested in examining the seasonality of microbe communities. In spring, blooms in phytoplankton occur due to the winter mixing, whereas in the summer months, the water becomes stratified, likely driving zooplankton closer to the surface. Seasonal changes are important for us to monitor and can help us understand the system in terms of long-term changes. The sheer abundance of microbes and zooplankton makes them important in biogeochemical cycling. Examining their community structure across time allows us to estimate carbon and nutrient cycling by looking at the metabolic potentials of the organisms. Seeing orcas on my cruise reminded me of the trophic energy transfer that occurs in the Salish Seas. While it may take millions of microbes and zooplankton being eaten by bigger fish until an orca comes by and eats them, studying the trends of lower trophic levels allows for a better understanding and appreciation of the entire ecosystem.
Invalid Scald ID.
A new video highlighting PMEL's acoustics research and eDNA innovations to study Blue Whales off the coast of Newport, Oregon has been released in the “PMEL at Work” YouTube series.
This video features PMEL’s Acoustic Program’s Bob Dziak and Angela Sremba discussing ongoing research to develop technology to better understand blue whales by simultaneously collecting environmental DNA (eDNA) samples and recording acoustic calls through deploying drifting buoys with underwater hydrophones.
Over the summer of 2021, PMEL and OSU CIMERS scientists field tested three passive-acoustic hydrophones to identify and locate blue and fin whales. These new smart hydrophones have an internal microprocessor that communicates the location of the buoys to shore. PMEL developed software for the microprocessor that will analyze the hydrophone data using a blue whale call detection algorithm. The goal for this algorithm is to register a positive blue call detection and transmit this positive detection information, as well as the position of the buoy, via satellite communication to shore to let scientists know when and where a blue whale call is recorded. When a call is detected on multiple buoys, the shore based scientists can then determine the location of the calling whale, and then inform the ship’s crew that a blue whale is nearby. The location of the whale will be correlated with the results from collected eDNA samples.
This past summer was a successful field test of the hydrophone buoys in anticipation for upcoming fieldwork in the summer of 2022 where PMEL will simultaneously collect acoustic recordings and eDNA samples of blue whales off the Oregon coast. This project will improve interpretation of eDNA results by using a time-series sampling method combined with acoustic detection for tracking and localizing highly migratory whale species in remote open ocean habitats.
This is a collaborative project between Oregon State University Marine Mammal Institute and Cooperative Institute for Marine Ecosystem and Resources Studies (CIMERS) and NOAA.
The PMEL at Work video series highlights ongoing research activities and projects supporting NOAA’s mission to understand changes in the global ocean and its impact on climate, weather and ecosystems.
On June 13, scientists aboard the NOAA Ship Ronald H. Brown set out on the West Coast Ocean Acidification Research Cruise to characterize conditions along the West Coast of North America and continue to build a unique time-series of carbon and hydrographic measurements in areas expected to be highly impacted by ocean acidification. Scientists have been collecting samples from CTDs, collecting plankton and water samples for genomics analysis, and conducting the first systematic regional survey of methane gas coming out of the thousands of seeps along the west coast.
The California Current System, running along the North American west coast from British Columbia to Baja California, is a region where seasonal upwelling brings nutrient- and carbon dioxide-rich and oxygen-poor waters to the surface. Increasing levels of carbon dioxide from upwelling and anthropogenic emissions, cause a series of chemical reactions that are ultimately increasing acidity in these waters. Because it is an area with high rates of primary production by phytoplankton, air-sea carbon dioxide exchange, and carbon export to the open ocean and sediments, it is particularly susceptible to the impacts of ocean acidification and hypoxia. Understanding the progression of ocean acidification in coastal areas in the context of these other natural processes is critical for developing management, mitigation, and adaptation strategies.
This 47-day research cruise brings together an international team of scientists from the United States, Canada, Mexico, Finland, and the Netherlands to measure acidity, temperature, oxygen, and chlorophyll from 16 transect lines stretching from British Columbia, Canada to San Diego, California. They also have deployed net tows to sample phytoplankton, zooplankton, and fish to analyze how the marine food web is being affected by acidified waters.
With data collected from this cruise, and previous ocean acidification cruises in this region, scientists are documenting the changing ocean acidification conditions and how they are impacting marine ecosystems against a backdrop of multiple stressors including warming and deoxygenation. In 2016, measurements from this cruise demonstrated, for the first time, that ocean acidification along the US Pacific Northwest coast is impacting the shells and sensory organs of some larval Dungeness crab and that pteropods sampled near the coasts of Washington and Oregon had shells 37 percent thinner than those in waters further offshore.
With the comprehensive approach taken on this year’s mission- combining detailed physical, chemical and biological measurements - we will not only better understand how our ocean is changing, but also test what new tools can be used to assess the future of these important marine ecosystems.
“A strong understanding of both the changes in chemistry and marine life allows us to make informed decisions to sustain the ecosystems, communities and industry members along the West coast,” says Libby Jewett, Director of NOAA’s Ocean Acidification Program which funds this research cruise.
Follow along scientists aboard with NOAA’s Ocean Acidification Program’s Twitter, Instagram and Facebook .
41 scientists from PMEL, including scientists from NOAA's cooperative institutes at the University of Washington's Joint Institute for the Study of the Ocean and Atmosphere (JISAO) and Oregon State University's Cooperative Institute for Marine Resources Studies (CIMRS), the National Research Council, graduate and undergraduate students are heading to the Ocean Sciences Meeting in San Diego to share their current research. Talks and posters cover a range of topics include saildrone research, ocean observing systems, marine heatwaves, Arctic, acoustics, Deep Argo, genetics and genomics, El Nino, hydrothermal vents, methane, nutrients, technologies, ocean carbon and data management.
The 2020 Oceans Science Meeting is the flagship conference for the ocean sciences and the larger ocean-connected community. As we approach the UN Decade of Ocean Science for Sustainable Development, beginning in 2021, it is increasingly important to gather as a scientific community to raise awareness of the truly global dimension of the ocean, address environmental challenges, and set forth on a path towards a resilient planet. The meeting is co-sponsored by the American Geophysical Union (AGU), the Association for the Sciences of Limnology and Oceanography (ASLO), and The Oceanography Society (TOS).
PMEL research groups that will be present at the conference are: Acoustics, Arctic including Innovative Technology for Arctic Exploration, Climate-Weather Interface, Earth-Ocean Interactions, EcoFOCI, Engineering, Genetics and Genomics, Global Tropical Moored Buoy Array, , Large Scale Ocean Physics, Ocean Carbon, Ocean Climate Stations, Pacific Western Boundary Currents, and Science Data Integration Group.