A pH unit is a measure of acidity ranging from 0-14. The lower the value, the higher the acidity of the environment. A shift in pH to a lower value reflects an increase in acidity.
The Chemistry
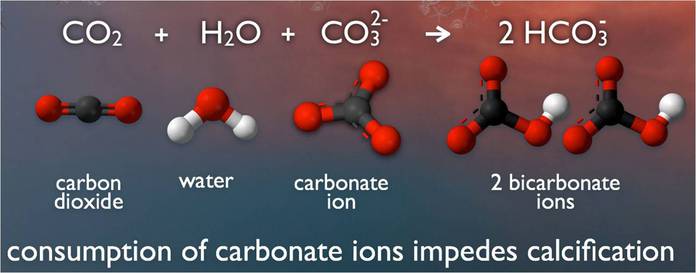
When carbon dioxide (CO2) is absorbed by seawater, chemical reactions occur that reduce seawater pH, carbonate ion concentration, and saturation states of biologically important calcium carbonate minerals. These chemical reactions are termed "ocean acidification" or "OA" for short. Calcium carbonate minerals are the building blocks for the skeletons and shells of many marine organisms. In areas where most life now congregates in the ocean, the seawater is supersaturated with respect to calcium carbonate minerals. This means there are abundant building blocks for calcifying organisms to build their skeletons and shells. However, continued ocean acidification is causing many parts of the ocean to become undersaturated with these minerals, which is likely to affect the ability of some organisms to produce and maintain their shells.
Since the beginning of the Industrial Revolution, the pH of surface ocean waters has fallen by 0.1 pH units. Since the pH scale, like the Richter scale, is logarithmic, this change represents approximately a 30 percent increase in acidity (see our pH primer web page for more information). Future predictions indicate that the oceans will continue to absorb carbon dioxide, further increasing ocean acidity. Estimates of future carbon dioxide levels, based on business as usual emission scenarios, indicate that by the end of this century the surface waters of the ocean could have acidity levels nearly 150 percent higher, resulting in a pH that the oceans haven’t experienced for more than 20 million years.
The Biological Impacts
Ocean acidification is expected to impact ocean species to varying degrees. Photosynthetic algae and seagrasses may benefit from higher CO2 conditions in the ocean, as they require CO2 to live just like plants on land. On the other hand, studies have shown that lower environmental calcium carbonate saturation states can have a dramatic effect on some calcifying species, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton. Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. Thus, both jobs and food security in the U.S. and around the world depend on the fish and shellfish in our oceans.
Pteropods
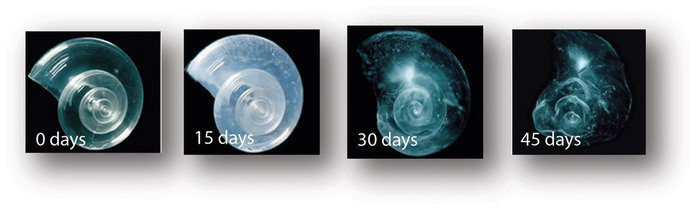
The pteropod, or “sea butterfly”, is a tiny sea creature about the size of a small pea. Pteropods are eaten by organisms ranging in size from tiny krill to whales and are a food source for North Pacific juvenile salmon. The photos below show that a pteropod’s shell dissolves over 45 day when placed in sea water with pH and carbonate levels projected for the year 2100. Photo credit: David Liittschwager/National Geographic Stock. Used with permission. All rights reserved. National Geographic Images.
Shellfish

In recent years, there have been near total failures of developing oysters in both aquaculture facilities and natural ecosystems on the West Coast. These larval oyster failures appear to be correlated with naturally occurring upwelling events that bring low pH waters undersaturated in aragonite as well as other water quality changes to nearshore environments. Lower pH values occur naturally on the West Coast during upwelling events, but a recent observations indicate that anthropogenic CO2 is contributing to seasonal undersaturation. Low pH may be a factor in the current oyster reproductive failure; however, more research is needed to disentangle potential acidification effects from other risk factors, such as episodic freshwater inflow, pathogen increases, or low dissolved oxygen. It is premature to conclude that acidification is responsible for the recent oyster failures, but acidification is a potential factor in the current crisis to this $100 million a year industry, prompting new collaborations and accelerated research on ocean acidification and potential biological impacts.
Photo: Freshly harvested oysters from Yaquina Bay, Oregon (Credit: NOAA)
Coral
Many marine organisms that produce calcium carbonate shells or skeletons are negatively impacted by increasing CO2 levels and decreasing pH in seawater. For example, increasing ocean acidification has been shown to significantly reduce the ability of reef-building corals to produce their skeletons. In a recent paper, coral biologists reported that ocean acidification could compromise the successful fertilization, larval settlement and survivorship of Elkhorn coral, an endangered species. These research results suggest that ocean acidification could severely impact the ability of coral reefs to recover from disturbance. Other research indicates that, by the end of this century, coral reefs may erode faster than they can be rebuilt. This could compromise the long-term viability of these ecosystems and perhaps impact the estimated one million species that depend on coral reef habitat.
Ocean Acidification: An Emerging Global Problem
Ocean acidification is an emerging global problem. Over the last decade, there has been much focus in the ocean science community on studying the potential impacts of ocean acidification. Since sustained efforts to monitor ocean acidification worldwide are only beginning, it is currently impossible to predict exactly how ocean acidification impacts will cascade throughout the marine food chain and affect the overall structure of marine ecosystems. With the pace of ocean acidification accelerating, scientists, resource managers, and policymakers recognize the urgent need to strengthen the science as a basis for sound decision making and action.